Improving compliance in Indian Pharma industry
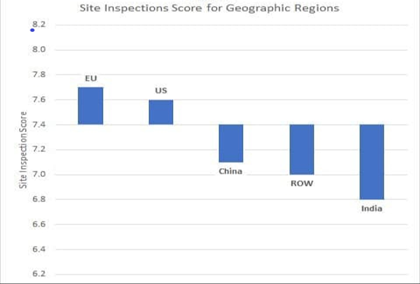
Improving compliance in Indian Pharma industry - Ganadhish Kamat USFDA scores each site inspected by them based on the inspection outcome. The site inspection score provides one measure of a site’s compliance to CGMP regulations. Report on the State of Pharmaceutical Quality for 2019 published by CDER, included a summary of site inspection scores by different regions of the world. This summary showed that the average inspection score across the globe was 7.4. While the average scores of the sites in EU (7.7) and US (7.6) were above the global average, the average site scores in China (7.1), ROW (7.0) and India (6.8) were less, with Indian average being the least. Indian sites also had large share of warning letters issued globally in last few years. The analysis of the warning letters issued to Indian sites since 2015 is given below – After going through individual warning letters it is clear that the concerns raised in the warning letters were largely related t
